Vacuole Coloring Techniques: Animal Cell Coloring Vaulcule
Animal cell coloring vaulcule – Visualizing vacuoles within animal cells, while often smaller and less prominent than those in plant cells, is crucial for understanding cellular processes. Different staining techniques, exploiting the chemical properties of vacuolar contents and the cell membrane, allow for effective visualization under various microscopy methods. The choice of technique depends on the specific research question and the desired level of detail.Various microscopy methods can be employed to visualize vacuoles after staining, each offering unique advantages.
Brightfield microscopy, while simple, often requires strong staining for effective visualization. Fluorescence microscopy, on the other hand, allows for greater specificity and sensitivity, particularly when using fluorescent dyes that target specific vacuolar components. Electron microscopy provides the highest resolution, enabling the detailed study of vacuolar structure and membrane properties, though sample preparation is more complex.
Vacuole Staining with Neutral Red
Neutral red is a vital dye commonly used to stain vacuoles in animal cells. It is a relatively safe dye, but standard laboratory safety precautions should always be followed. The dye accumulates within vacuoles due to their slightly acidic internal pH.
- Preparation: Prepare a dilute solution of neutral red (typically 0.1% in water or a suitable buffer). The exact concentration may need to be adjusted depending on the cell type and desired staining intensity.
- Sample Preparation: Prepare a cell slide. This may involve culturing cells on a coverslip or using a prepared cell smear. Ensure the cells are appropriately fixed (e.g., using a gentle fixative like paraformaldehyde) to maintain their structure and prevent dye leakage.
- Staining: Gently apply the neutral red solution to the cell slide, ensuring complete coverage. Allow the stain to incubate for a specified period (e.g., 5-15 minutes), monitoring the staining intensity under a microscope. Excessive staining can obscure details.
- Washing: Gently rinse the slide with distilled water or buffer to remove excess stain. Avoid harsh washing, which may dislodge cells or damage the sample.
- Mounting: Mount the slide with a coverslip using a suitable mounting medium (e.g., aqueous mounting medium) and observe under a microscope.
Safety Precautions: Always wear appropriate personal protective equipment (PPE), including gloves and eye protection, when handling stains. Dispose of used stain and other materials according to laboratory safety protocols. Neutral red is considered a low-hazard dye, but skin and eye contact should be avoided.
Comparison of Neutral Red and Lysotracker Staining, Animal cell coloring vaulcule
This experiment compares the effectiveness of neutral red and Lysotracker Red DND-99, a fluorescent dye that specifically targets acidic organelles like lysosomes (which are functionally related to vacuoles). The experiment aims to determine which dye provides better visualization of vacuoles in a specific cell type (e.g., macrophages).
- Hypothesis: Lysotracker Red will provide more specific and brighter staining of vacuoles compared to neutral red due to its targeted binding to acidic compartments.
- Materials: Cell culture (e.g., macrophages), neutral red solution (0.1%), Lysotracker Red solution (as per manufacturer’s instructions), microscope slides, coverslips, mounting medium, brightfield and fluorescence microscopes.
- Procedure: Two sets of identical cell slides are prepared. One set is stained with neutral red, and the other with Lysotracker Red, following the manufacturer’s instructions for each dye. Both sets are then observed under both brightfield and fluorescence microscopy (if applicable).
- Data Collection: Microscopic images are captured at various magnifications. The intensity and specificity of staining are assessed for each dye by visually comparing the images and quantifying the number of stained vacuoles per cell. Image analysis software can be used to quantify fluorescence intensity.
- Analysis: The results are analyzed to determine which dye produced more intense and specific staining of vacuoles. Statistical tests (e.g., t-test) can be used to compare the mean number of stained vacuoles between the two groups.
Vacuole Size and Distribution
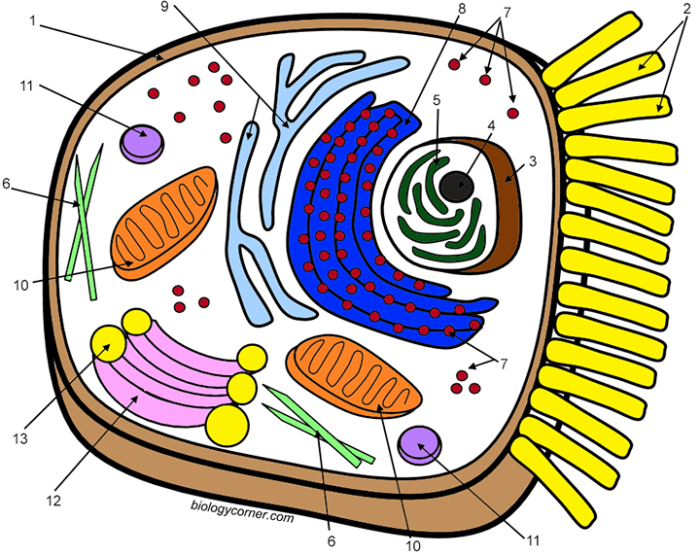
Animal cells, unlike their plant counterparts, generally possess smaller and more numerous vacuoles. The size and distribution of these vacuoles are not static; they are dynamic structures that reflect the cell’s physiological state and function. Several factors influence this variability, leading to a wide range of vacuole characteristics across different animal cell types.The size and number of vacuoles in an animal cell are primarily determined by the cell’s metabolic activity and its role within the organism.
Cells involved in secretion, such as those in glands, often exhibit a larger number of smaller vacuoles to efficiently manage the transport and storage of secretory products. Conversely, cells with less secretory activity might have fewer, larger vacuoles, or even lack prominent vacuoles altogether. The availability of water and other solutes also plays a crucial role, influencing the vacuole’s tonicity and overall size.
Furthermore, the age and health of the cell impact vacuole morphology; aging or stressed cells might show altered vacuole size and distribution as a consequence of cellular damage or dysfunction.
Factors Influencing Vacuole Size and Number
Several key factors contribute to the variation in vacuole size and number observed in animal cells. These factors include the cell type, its metabolic activity, the overall cellular hydration status, and the cell’s stage in its life cycle. For instance, macrophages, immune cells responsible for engulfing foreign particles, frequently exhibit large vacuoles containing ingested material. In contrast, muscle cells typically possess a relatively small number of small vacuoles, reflecting their primary function in contraction.
Cellular stress or disease can also significantly alter vacuole characteristics, sometimes leading to an increase in size or number as a result of cellular dysfunction.
Vacuole Characteristics in Different Animal Cell Types
The differences in vacuole size and distribution are readily apparent when comparing various animal cell types. For example, epithelial cells lining the digestive tract often contain numerous small vacuoles involved in the absorption and transport of nutrients. In contrast, fat cells (adipocytes) are characterized by a single, large vacuole that occupies most of the cell’s volume, storing lipids.
Neurons, responsible for transmitting nerve impulses, generally have relatively few and small vacuoles. These variations reflect the specialized functions of each cell type and the different demands placed upon their intracellular trafficking and storage systems.
Vacuole Size and Distribution Data
| Cell Type | Average Vacuole Size (µm) | Number of Vacuoles per Cell | Notes |
|---|---|---|---|
| Macrophage | 5-15 | Variable, often numerous | Size depends on ingested material |
| Adipocyte | >50 | 1 (dominant) | Single, large vacuole for lipid storage |
| Hepatocyte (Liver Cell) | 2-5 | Numerous | Involved in detoxification and metabolism |
| Skeletal Muscle Cell | <2 | Few | Relatively small and infrequent |
Vacuole Contents and Function
Animal cell vacuoles, while smaller and less prominent than those in plant cells, play a crucial role in various cellular processes. They are not simply empty spaces; instead, they are dynamic compartments containing a diverse array of substances that contribute significantly to the cell’s overall function and health. Understanding the contents and their functions provides valuable insight into the complexity of animal cell biology.Animal cell vacuoles contain a variety of substances, depending on the cell type and its current metabolic state.
These substances are often temporarily stored or transported within the vacuole before being used elsewhere in the cell. The contents can range from water and ions to larger molecules like proteins and lipids. Furthermore, waste products and even cellular debris can also be found within these organelles. The precise composition of a vacuole’s contents is constantly changing as the cell’s needs fluctuate.
Vacuole Contents: A Diverse Inventory
Vacuoles act as temporary storage sites for various molecules. For example, they may store excess water, helping to regulate the cell’s osmotic balance. Ions such as calcium and potassium are frequently stored in vacuoles, ready to be released when needed for cellular signaling or other metabolic processes. Many proteins are also temporarily held within vacuoles during their transport to their final destinations.
Lipids and other metabolites are often stored as well, providing a ready source of energy or building blocks for various cellular components. Finally, vacuoles also participate in the sequestration of waste products and potentially harmful substances, preventing them from interfering with other cellular functions.
Vacuole Contents and Cellular Processes
The contents of animal cell vacuoles directly impact several key cellular processes. For instance, the regulated release of calcium ions from vacuoles triggers muscle contraction. Similarly, the storage and release of other signaling molecules within vacuoles plays a vital role in cell communication and response to stimuli. The breakdown of waste products within vacuoles is crucial for maintaining cellular health and preventing the accumulation of toxic substances.
In some cell types, vacuoles participate in the process of autophagy, where damaged organelles are broken down and recycled. The storage of nutrients within vacuoles also provides a reserve supply of energy and building blocks for the cell, particularly during periods of nutrient scarcity.
Diagram of a Vacuole’s Internal Components
Imagine a roughly spherical vacuole, represented in light beige. Within this vacuole, several components are depicted. Small, dark blue circles represent water molecules. Slightly larger, light green circles represent lipid droplets. Purple ovals represent proteins, some larger and some smaller to indicate size variability.
Understanding the intricacies of an animal cell, particularly coloring the vacuole, can be a surprisingly engaging activity. This detailed process is reminiscent of the complexity found in other visual puzzles, such as the animal captivity coloring sheet puzzle , which also requires careful attention to detail. Returning to the cellular level, accurately depicting the vacuole’s size and location within the animal cell truly highlights the cell’s overall structure.
Several small, bright orange spheres depict calcium ions. Finally, a few irregularly shaped, dark brown structures represent waste products. These various components are not neatly organized; instead, they are distributed somewhat randomly throughout the vacuole’s interior, reflecting the dynamic nature of its contents. The overall appearance is one of a constantly shifting mixture of substances, highlighting the vacuole’s role as a temporary storage and processing center within the animal cell.
Vacuole Dynamics and Changes
Plant and animal cells exhibit dynamic changes in vacuole size and number, reflecting the cell’s physiological state and environmental conditions. These alterations are not random but are tightly regulated processes crucial for maintaining cellular homeostasis and responding to internal and external stimuli. Understanding these dynamic aspects provides valuable insight into cellular function and adaptation.Vacuole size and number are highly responsive to cellular hydration status and metabolic activity.
For instance, in plant cells, large central vacuoles contribute significantly to turgor pressure, maintaining cell rigidity. Water uptake leads to vacuole expansion, increasing cell volume. Conversely, water loss results in vacuole shrinkage and cellular plasmolysis. In animal cells, while smaller and more numerous, vacuoles still respond to osmotic changes, though their contribution to overall cell volume is less pronounced than in plants.
Changes in metabolic activity, such as increased nutrient availability, can also stimulate vacuole biogenesis and growth to accommodate storage needs. Conversely, during nutrient deprivation or cellular stress, vacuoles may undergo autophagy, a process where cellular components are degraded and recycled, resulting in a reduction in vacuole size and number.
Vacuole Formation and Degradation Mechanisms
Vacuole formation, or biogenesis, involves the fusion of smaller vesicles derived from the endoplasmic reticulum and Golgi apparatus. These vesicles transport materials, such as water, ions, and storage compounds, to the growing vacuole. The precise mechanisms regulating vesicle trafficking and fusion are complex and involve specific proteins, including Rab GTPases and SNARE proteins, which mediate vesicle targeting and membrane fusion.
The size and number of vacuoles formed are influenced by the rate of vesicle delivery and fusion.Vacuole degradation, on the other hand, often occurs through autophagy. In this process, portions of the cytoplasm, including damaged organelles or excess storage materials, are enclosed within double-membrane vesicles called autophagosomes. These autophagosomes then fuse with vacuoles (or lysosomes in animal cells), delivering their contents for degradation by hydrolytic enzymes present within the vacuole lumen.
The resulting breakdown products can be recycled for cellular use. The regulation of autophagy is complex and involves signaling pathways sensitive to nutrient availability and cellular stress levels. For example, under starvation conditions, autophagy is upregulated to provide essential nutrients by breaking down cellular components.
Factors Affecting Vacuole Function
The efficient functioning of vacuoles depends on a variety of factors that can influence their formation, maintenance, and degradation.
- Nutrient Availability: Abundant nutrients stimulate vacuole growth for storage, while nutrient deprivation triggers autophagy and vacuole shrinkage.
- Water Availability: Water uptake causes vacuole expansion, contributing to turgor pressure (in plants) and cell volume regulation. Water stress leads to vacuole shrinkage and potential cellular damage.
- pH Levels: The internal pH of the vacuole is crucial for enzyme activity and storage of various compounds. Disruptions in pH can impair vacuole function.
- Ion Concentrations: The vacuole plays a role in ion homeostasis. Imbalances in ion concentrations can affect vacuole size, membrane stability, and overall cellular function.
- Cellular Stress: Stressful conditions, such as heat shock, oxidative stress, or pathogen infection, can trigger changes in vacuole dynamics, potentially leading to autophagy or alterations in vacuole membrane permeability.
- Genetic Factors: Genes encoding proteins involved in vacuole biogenesis, trafficking, and degradation influence vacuole size, number, and function. Mutations in these genes can lead to vacuolar defects.
Key Questions Answered
What are the limitations of using only one staining technique to visualize vacuoles?
Using a single staining technique may not reveal the full complexity of vacuole structure or contents. Different dyes bind to different molecules, potentially obscuring certain features or providing an incomplete picture.
How does the pH of the staining solution affect vacuole visualization?
The pH of the staining solution can significantly impact the dye’s binding affinity to vacuolar components. Optimal pH must be determined for each dye to ensure effective staining and prevent artifacts.
Can vacuoles be visualized in living cells without staining?
Yes, certain advanced microscopy techniques, such as phase-contrast or differential interference contrast microscopy, allow for visualization of vacuoles in living cells without the need for staining, though detail may be limited.












